Global Information for Public Health Transformation (GIPHT) Initiative
Global Collaboration to Address the COVID-19 Pandemic and Future Public Health Challenges
LAUNCHED March 2020
Refugee Protected Personal Health Information Project Overview
GIPHT Pilot Project: A PERSON-CENTERED APPROACH TO INTERNATIONAL HEALTH RECORD INTEROPERATION FOR REFUGEES FROM UKRAINE AND OTHER NATIONS (Launched February 2022)
The Inalienable Human Right to Health
We believe refugees fleeing their home countries, and leaving so much behind, carry with them the inalienable human right to health. This requires that they take their health information with them wherever they may go. Doing so ensures that they can be more confident of receiving safe and effective healthcare - which refugees often need urgently – and empowers them to advance their health and the health of others, while also supporting public health agencies in their missions.
DRAFT Pilot Project Initial Vision Document (September 2022)
Vaccination Project Overview
GIPHT Project: TOWARDS ONE GLOBAL STANDARD FOR DIGITAL PROOF OF COVID-19 VACCINATION (Launched March 2020)
Why Should We have a Global Common Digital way to Show Proof of COVID-19 vaccination?
Every day more people across the globe are receiving vaccinations to protect them from COVID-19. Any one of us may need to show proof that we have received these vaccines, which ones and on what dates we received them. Such proof could be necessary when we travel abroad, and sometimes locally to access buildings and events. We may also need to show this information to our health professionals if they don’t already have this.
Countries in Europe and certain states in the U.S. have raced to create something like a vaccination certificate app that carries information about your vaccination status that can be shared with relevant authorities. The problem is that countries have chosen different data structures and codes to document the COVID-19 vaccinations given in their country, and companies are collecting and storing vaccination data in different ways on apps and databases. And, in some states in the U.S., such proof of vaccination is still a paper card that is not secure. This is making it increasingly difficult for vaccination data to be exchanged between countries, which may be needed for authorization to travel or to attend a university.
Clearly, a single global vaccine data standard should be developed and implemented by all developers of apps to demonstrate proof of Covid-19 vaccination!
What is the GIPHT Group Doing to Promote the Use of a Single Global COVID-19 Data Standard for Digital Proof of Vaccine Administration?
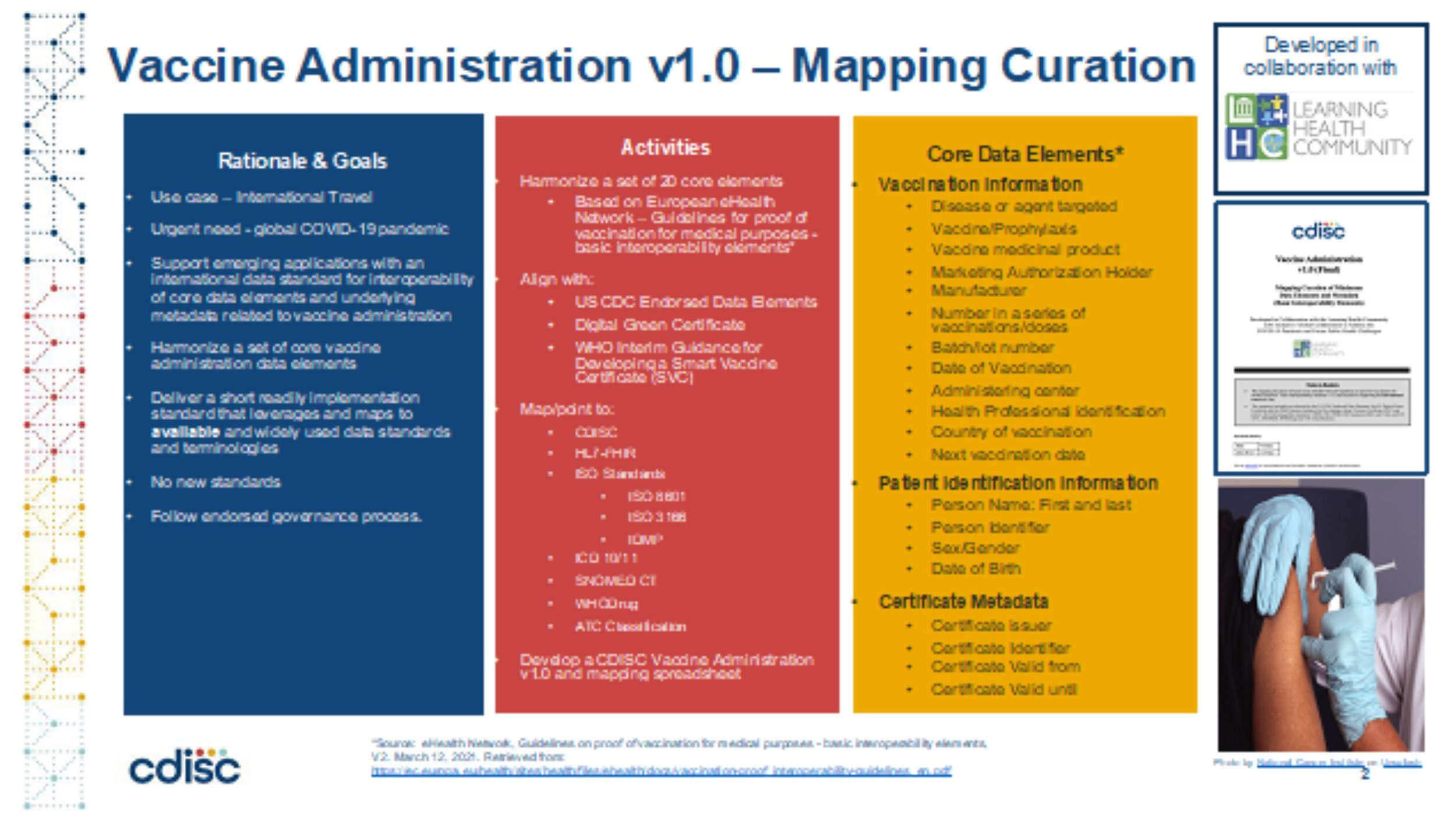
The Learning Health Community’s Global Initiative for Public Health Transformation (GIPHT) Initiative and the global standards development organization, Clinical Data Interchange Standards Consortium (CDISC) partnered to develop the V1.0 Vaccine Administration Standard.
An international standard for the data that documents the administration of COVID-19 vaccinations was published in June 2021. This standard built on vaccination data structures that had been developed by the World Health Organisation, by the European Commission and by other bodies. It harmonises their different data structures into a single universal format. This standard has been published, is openly available, free to all. We now need to encourage everyone around the planet to utilise this standard rather than having our data stored in different incompatible formats by different countries and on different apps.
If you would like to download a copy of the standard yourself, you can find it here.
Please visit: VaccineAdministration.org
Consensus data Standards
Considering the urgency during the global COVID-19 pandemic, the Learning Health Community’s Global Initiative for Public Health Transformation (GIPHT) Initiative and the global standards development organization, Clinical Data Interchange Standards Consortium (CDISC) have partnered to develop the V1.0 Vaccine Administration Standard.
The core elements were identified by referencing a) the eHealth Network Guidelines for proof of vaccination for medical purposes - basic interoperability elements; b) corresponding CDC-endorsed data elements posted by the American Immunization Registry Association; c) the EU Digital Green Certificate documentation; and d) the WHO Interim Guidance for Developing a Smart Vaccine Certificate (SVC).
These core data elements were then mapped/pointed to prevailing global standards from Clinical Data Interchange Standards Consortium (CDISC); Health Level 7 (HL7) and the International Standards Organization (ISO), ICD 10/11. SNOMED CT, WHODrug and the ATC Classification to create the Vaccine Administration Standard v1.0. The result can effectively ensure that data can flow efficiently and comprehensively among various applications that implement this standard. Global adoption will support safe and efficient global travel.
This standard thus aligns with the WHO recommendations and provides additional metadata to ensure interoperability among apps developed by different companies.
The result can effectively ensure that data can flow efficiently and comprehensively among various applications that implement this standard.
Please visit: VaccineAdministration.org
Figure 1: Vaccine Administration v1.0 Project Summary
Global adoption of this standard by app developers will support safe and efficient global travel for all!
This will also support other use cases such as admission to events and universities.
Communications
Guides
CDISC COVID-19 Therapeutic Area and Vaccine Administration Standards Guides
Website: VaccineAdministration.org
Press Releases
Press Release 20 April 2021 “Global Public Health Data Standard to Enable Vaccine Certification Interoperability”: CDISC, Business Wire, Yahoo! Finance, Bloomberg
Press Release 22 June 2021 “Newly Released Global Public Health Data Standard to Enable Vaccine Administration Data Interoperability”: CDISC, Business Wire, Yahoo! Finance, Bloomberg
Public Comments
Opportunity to Comment by 20 May 2021, Open to the Public
Supplemental Materials
Global Vaccine Administration Standard Overview DRAFT Presented 8 June 2021
Public Communication: “A Standard for COVID-19 Vaccination Data” 8 September 2021
Presentation at the United States National Vaccine Advisory Committee (NVAC) Meeting: “Vaccine Administration Standard” 11 February 2022
Research and Publications
Publications
Peer-Reviewed Paper (November 2020): “Addressing the Covid‐19 pandemic and future public health challenges through global collaboration and a data‐driven systems approach”
Vision Document: “Learning Beyond Boundaries: Linking Global Data to Life-Saving Action in an Era of Pandemics”
Leadership
Multi-National Organizers (Italy, Spain, the United Kingdom, and the United States of America)
Luigi Bertinato, MD, PhD (ITALY) Head of the Office of the Scientific Secretariat to the President of the Italian National Institute of Health, Ministry of Health; Formerly WHO in Africa; DG Santè of the European Commission
Dave Evans, MS (USA) President and CEO, CDISC
Rhonda Facile, MS (USA) Vice President of Partnerships and Development, CDISC
Charles P. Friedman, PhD (USA) Chair of the Department of Learning Health Sciences, Josiah Macy Jr. Professor of Medical Education at the University of Michigan Medical School; Professor of Information and Public Health; Founder, Learning Health Community; Former Deputy Director and Chief Scientific Officer, U.S. Office of the National Coordinator for Health IT
Esther Gil Zorzo (SPAIN) President of Educatec Foundation; Diabetes Coordinator in HM Hospitals; Former President of the FEAED (National Federation for Diabetes Educators); Member of the Executive Committee of the FEND (Federation European Nurses Diabetes)
Dipak Kalra, PhD, FRCGP, FBCS (UK) President of The European Institute for Innovation through Health Data (i~HD)
Rebecca D. Kush, PhD (USA) President, Catalysis; Chief Scientific Officer for Elligo Health Research; Founder and President Emeritus of the Clinical Data Interchange Standards Consortium (CDISC); Director on the Board, Learning Health Community; Former HL7 Board Member and Member of U.S. Health IT Standards Committee
Brian Martin, MD (USA) Principal Digital Health Analyst, The MITRE Corporation
Pablo Rivero (SPAIN) Senior Health and Public Sector Advisor for Everis / NTT Data; Member of the Digital Health Roster of Experts, World Health Organization; Former Director General for the Agency for Quality and Innovation of the National Health System of Spain
Francisco Ros, PhD (SPAIN) President, First-Tech Engineering; Former Secretary of State for Telecommunications and the Information Society in the government of Spain; former Qualcomm Board Member
Joshua C. Rubin, JD, MBA, MPH, MPP (USA) Program Officer for Learning Health System Initiatives at the Department of Learning Health Sciences, University of Michigan Medical School; CEO of the Learning Health Community
Paolo Stocco (ITALY) Executive Board Member, EuroHealthNet, a not-for-profit partnership of organizations, agencies and statutory bodies working on public health, disease prevention, promoting health, and reducing inequalities
Douglas Van Houweling, PhD (USA) Professor Emeritus of Information, School of Information; Professor Emeritus in Service of Learning Health Sciences, University of Michigan Medical School
William A. Yasnoff, MD, PhD (USA) Adjunct Professor, Biomedical Informatics and Data Science, Johns Hopkins University; Managing Partner, National Health Information Infrastructure Advisors; Founder and President, Health Record Banking Alliance; Former Senior Advisor, National Health Information Infrastructure, U.S. Department of Health and Human Services
Contacts:
Rebecca D. Kush, PhD | rkush@catalysisresearch.com
Joshua C. Rubin, JD, MBA, MPH, MPP | Josh@JoshCRubin.com
BACKGROUND
The COVID-19 global pandemic has already taught us that the greatest public health challenges of our generation will show no respect for national boundaries, will impact lives and health of people of all nations, and will affect economies and quality of life in unprecedented ways. The types of rapid learning envisioned to address COVID‐19 and future public health crises require a systems approach that enables sharing of data and lessons learned at scale. Agreement on a systems approach augmented by technology and standards will be foundational to making such learning meaningful and to ensuring its scientific integrity. We formed a multi-national group focused on fighting current and future pandemics by leveraging a such data-driven systems approach anchored in the principles embodied by the multi-stakeholder consensus Core Values for Learning Health Systems.
The multi-national collaboration we formed was initially guided by experts from three countries--Spain, Italy, and the United States--all of which have experienced serious challenges during the COVID-19 pandemic. Our mission, as an initiative of the Learning Health Community, is to design and explore a systems-based approach to COVID-19 data management to inform real-time and strategic decisions during a pandemic or other public health emergencies.
We recognize that a pandemic is not merely a disease; it is a global public health emergency that requires meaningful, responsible data sharing and multi-stakeholder, trans-disciplinary, cross-sector, and cross-border collaboration. Further, some experts see the pressing challenge our world faces as a syndemic, instead of as a pandemic, to refer to the aggregation effect of the COVID‐19 to other diseases, mainly those of a chronic nature for socially vulnerable groups, anchored in deeply embedded social determinants underpinning patterns of inequity and inequality across societies that themselves demand urgent attention and systemic transformation. Our approach to realizing sustainable change embraces multi-national collaboration; when pathogens cross boundaries, the information to manage the disease must see no boundaries.
Multi-National Working Meetings
Working Meeting – International Standard for Vaccine Administration Data
Part I: 18 February 2021, 10:30-12:00 EST/16:30-18:00 CET
Contact: Rebecca D. Kush, PhD | rkush@catalysisresearch.com
Contact: Joshua C. Rubin, JD, MBA, MPH, MPP | Josh@JoshCRubin.com
Multi-National Working Meeting Invitation
Agenda Overview
Introductions
Urgency and Goals of the Proposed Project
International Consensus Towards Harmonizing Vaccine Administration Data
Current Landscape and Scope of Project
CDISC INITIAL DRAFT of Core Data Elements for Vaccine Administration Record Information
Questions
Breakout Groups for Discussion of Core Data Elements
Comments from Breakout Groups
Actions and Next Steps
Presentation: “Vaccination Documentation: International Data Standards as an Essential Foundation” (Part I)
Meeting Notes and Consensus Deliverables
Part II: 22 March 2021, 12:00-13:30 EDT/17:00-18:30 CET
Contact: Rebecca D. Kush, PhD | rkush@catalysisresearch.com
Contact: Joshua C. Rubin, JD, MBA, MPH, MPP | Josh@JoshCRubin.com
Presentation: “Vaccination Documentation: International Data Standards as an Essential Foundation” (Part II)
Meeting Notes and Consensus Deliverables
Mobile Apps (Beta)
Free, Global, Universal, Interoperable, Open Source, Mobile Apps (Beta):
Download Mobile App for iOS and Android (NOT an Epic Rickroll)
Download Meta Plugin for Facebook, Instagram, and WhatsApp (Definitely NOT a Rickroll)
Collaborators
CDISC
CDISC is a global non-profit charitable organization with over 500 member organizations from 30 countries. CDISC is incorporated as a 501(c)3 in the state of Massachusetts with an office in Austin, Texas and employees based in the US and Europe. CDISC Europe Foundation is based in Brussels, Belgium. CDISC Coordinating Committees (3Cs) strengthen international relationships in the EU, Japan, Asia Pacific and Korea and CDISC User Networks are located in Asia, Africa, EU, North America. These Networks discuss standards updates and developments, share implementation and learning experiences, participate in public review, and circulate feedback and new ideas to CDISC. CDISC standards are required for electronic submissions of study data to the US FDA and Japan’s PMDA and are recommended by China’s NMPA regulators. In the European Union regulators, in rare instances where subject level data is requested, they also recommend the use the CDISC standards. EMA also incorporated CDISC requirements for eSource Data Interchange into their field auditor guidance. EMA and PMDA have provided input into the development of CDISC Therapeutic Area Standards through the Coalition For Accelerating Standards and Therapies (CFAST), which also included the Critical Path Institute and TransCelerate. CDISC Europe Foundation has participated in a number of projects of the Innovative Medicines Initiative (IMI) in Europe, including EHR4CR, eTRIKS, Conect4children (a collaborative network for European Clinical trials) and DRAGON, (a consortium to secure AI imaging-based diagnosis, stratification, follow-up, and preparedness for coronavirus pandemics). CDISC has also provided courses on the CDISC standards to IMI members. In the United States, CDISC has formed alliances across the National Institutes of Health (NIH) including a long-standing collaboration with the National Cancer Institute (NCI); National Institute of Allergy and Infectious Diseases (NIAID), which has used CDISC standards for pharmacovigilance studies and meta-analyses and requires them for new HIV research protocols; the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), which was part of the consortium that developed CDISC’s polycystic kidney disease consortium referenced above; the National Institute of Neurological Disorders and Stroke (NINDS), which has contributed common concepts to our Therapeutic Area (TA) standard development in collaboration with NCI; and The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), which developed pediatric controlled terminologies managed by the NCI’s Enterprise Vocabulary Services (EVS).